
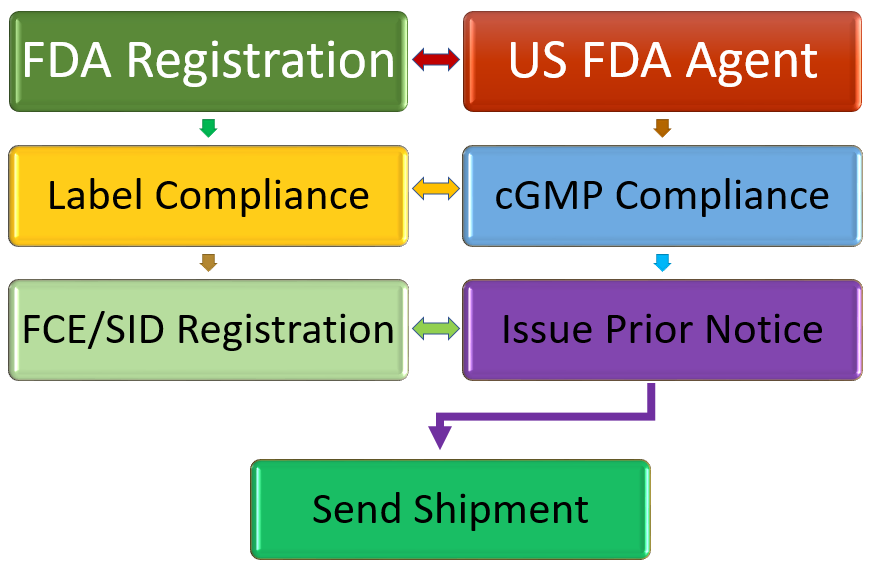
US FDA Requirements for foreign food facility
The FDA requirement for sending a food product to the USA are
- Register FDA
- US FDA Agent
- Label compliance
- Comply with cGMP requirements
- If acidified or low acid food, FCE Registration
- Issue prior notice before each shipment
Quick links
LIBERTY MANAGEMENT GROUP LTD.
75 Executive Drive, Suite 114
Aurora, Illinois, USA - 60504
Phone : +1 (630) 270-2921
Fax : +1 (815) 986-2632
E-mail : info@fdahelp.us
